Regulatory Guidance, Compliance Strategies, and Expert Insights for Regulated Industries
Navigating the complexities of FDA regulations, ISO standards, and evolving compliance frameworks requires more than just awareness — it demands expertise, insight, and reliable guidance. At Quality Solutions Now, our News and Advice hub delivers authoritative content designed to help medical device manufacturers, pharmaceutical firms, biotech companies, and combination product developers stay informed and proactive. From project management best practices to risk mitigation strategies, our articles and resources reflect the depth of experience behind our proprietary qsnFLOW™ methodology. Whether you’re preparing for an audit, launching a new product, or optimizing your quality systems, this is your trusted source for actionable, industry-specific knowledge that helps you reduce risk, ensure compliance, and drive operational excellence.
RAPS Convergence 2025: What to Expect at the Premier Regulatory Affairs Event
Starting today, October 6th, and ending on October 7th, regulatory [...]
FDA’s New Guidance on “Alternative Tools” for Manufacturing Facility Assessments: What Life Sciences Companies Need to Know
The FDA issued a new guidance on the use of [...]
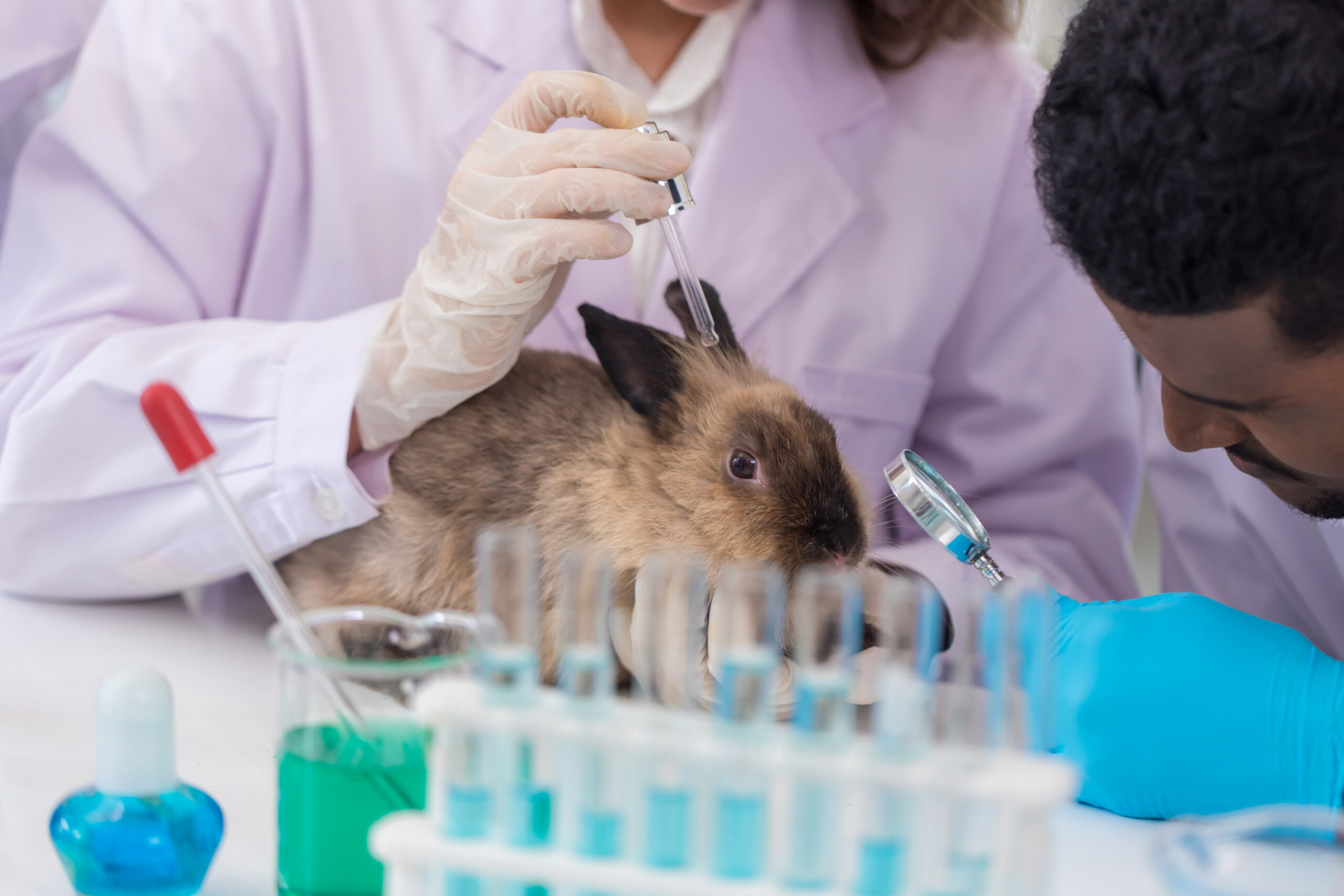
FDA to Phase Out Animal Testing Requirements, Promising Accelerated Cures and Smarter Drug Development
In April 2025, the U.S. Food and Drug Administration (FDA) [...]
FDA Issues Draft Guidance on 510(k) Transfer Protocols for Biotech and MedTech Firms
In June 2025, the U.S. Food and Drug Administration (FDA) [...]
Trump Grants Two-Year Delay for EtO Compliance: What It Means for Medical Device Manufacturers
On July 17, 2025, President Donald Trump issued an executive [...]
FDA Launches Communications Pilot to Accelerate High-Risk Medical Device Recall Notifications
In a move aimed at strengthening patient safety and enhancing [...]