Regulatory Guidance, Compliance Strategies, and Expert Insights for Regulated Industries
Navigating the complexities of FDA regulations, ISO standards, and evolving compliance frameworks requires more than just awareness — it demands expertise, insight, and reliable guidance. At Quality Solutions Now, our News and Advice hub delivers authoritative content designed to help medical device manufacturers, pharmaceutical firms, biotech companies, and combination product developers stay informed and proactive. From project management best practices to risk mitigation strategies, our articles and resources reflect the depth of experience behind our proprietary qsnFLOW™ methodology. Whether you’re preparing for an audit, launching a new product, or optimizing your quality systems, this is your trusted source for actionable, industry-specific knowledge that helps you reduce risk, ensure compliance, and drive operational excellence.
Adapting to FDA Shifts: How to Stay Ahead
Navigating FDA Changes: What Life Sciences Professionals Need to Know [...]
Navigating the Complexities of Combination Product Regulations and Patents
Navigating Regulatory and Patent Challenges in the Combination Products Space [...]
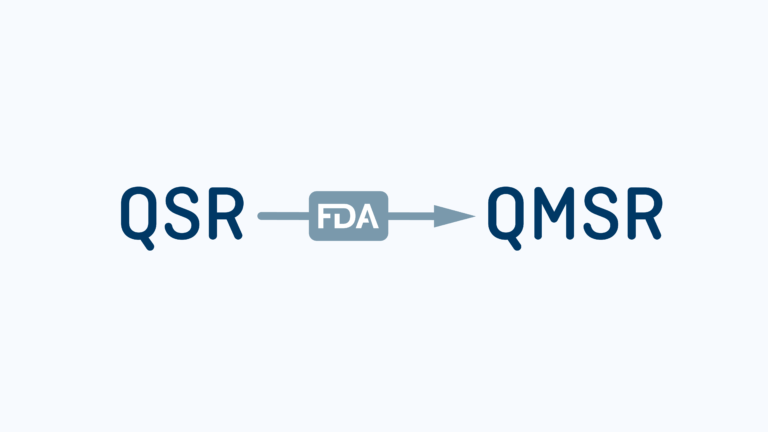
Getting QMSR Ready: Key Changes and Steps to Prepare for the February 2026 Deadline
Prepare Your Quality Management System for QMSR Compliance by February [...]
Tips for Hiring Specialty Expertise Consultants
Outsourcing with Confidence: A Guide to Successfully Hiring Specialty Expertise [...]
Your Guide to the 2024 Combination Products Summit: Agenda, Insights, and What to Anticipate
Agenda and Expectations for the 2024 Combination Products Summit: Navigating [...]
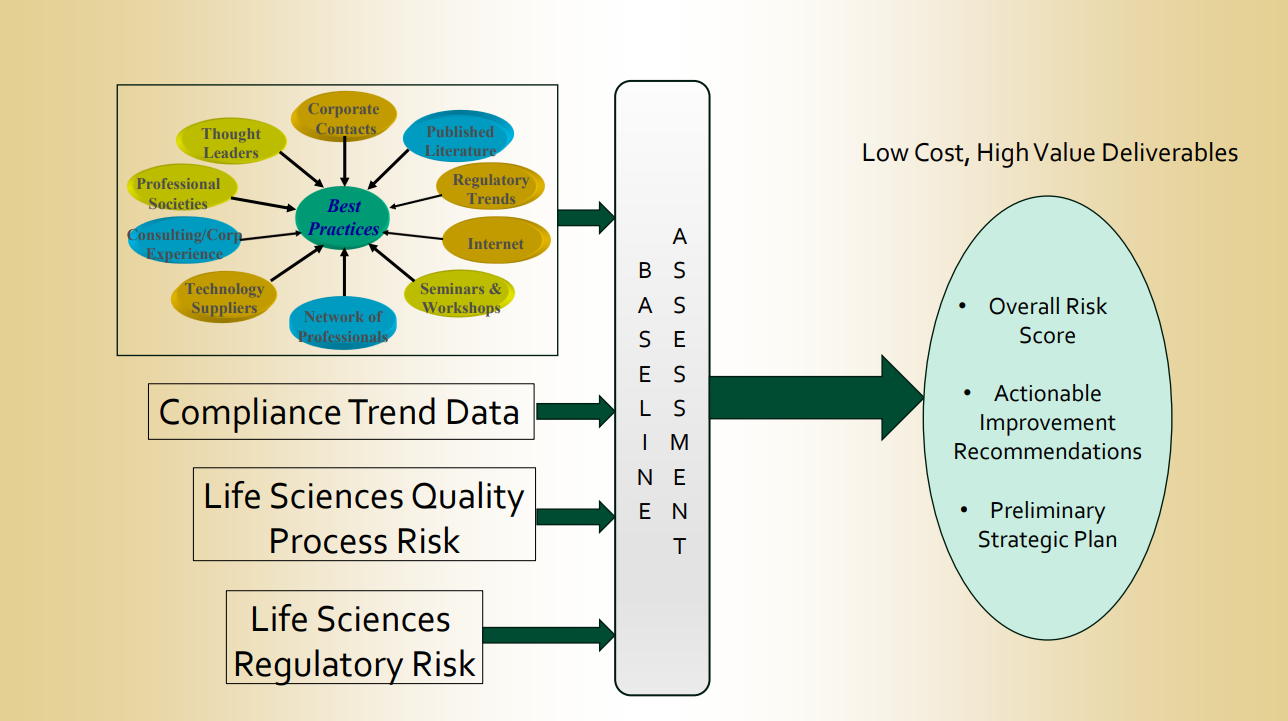
ComplyFDA Presents: Baseline Assessment
Enhancing Quality Processes: ComplyFDA’s Insightful Approach to Baseline Assessments Recently, [...]